Blog
June 26, 2025
How to Create a Requirements Traceability Matrix — with Examples
Security & Compliance,
Application Lifecycle Management
Creating a high-quality product that meets stakeholder expectations, regulatory compliance, and federal cybersecurity standards requires a high level of traceability throughout the development lifecycle. You must ensure requirements are met, tests are run, and issues are resolved with complete, auditable reporting that ties all your artifacts and their dependencies together.
The best way to achieve this is by creating a requirements traceability matrix (RTM).
👀 Read the guide: What is a requirements traceability matrix >>
While you may be tempted to create your traceability matrix in Microsoft Excel, an automated requirements management tool is a more effective, efficient, and dependable solution. In this guide, we’ll explore how to create a requirements traceability matrix, show RTM examples, and review the benefits of using a dedicated traceability tool.
Table of Contents
- 8 Steps to Create a Requirements Traceability Matrix
- 3 Challenges of Not Using Traceability Matrix Tools
- Why Requirement Traceability Tools are Better Than Spreadsheets
- Video Tutorial: Sample Traceability Matrix in Perforce ALM
- 9 Benefits of Creating Your Traceability Matrix with Perforce ALM
- Create a Free Traceability Matrix with Perforce ALM
 Back to top
Back to top
8 Steps to Create a Requirements Traceability Matrix
To build your requirements traceability matrix you must first create the template, or shell, of your matrix. This is where you'll determine what you want to trace, document your motives, and collect the necessary documents.
1. Define Your Goals
Your first step when creating a traceability matrix — whether you're using Excel or a dedicated requirements management tool — is to define your goals.
Ask yourself: What do I want to my traceability matrix to deliver?
Common goals include:
- Meeting compliance requirements
- Verifying that all requirements are tested and passed before release
- Identifying which tests or issues are affected when a requirement changes
Setting clear goals early on will help you determine the structure, tooling, and reporting needs of your matrix.
2. Gather and Identify Your Artifacts
Based on your goals, gather and label the most recent versions of your requirements documents and testing artifacts. Use unique and consistent IDs to prevent ambiguity and enable accurate linking, whether by the system or a human. These IDs should not change if your requirements are reordered nor should they be reused or deleted.
At its most basic, a traceability matrix should include:
- Requirements (functional and non-functional)
- Tests
- Test results
- Issues or defects
As you run test cases and log issues, carefully document outcomes and test statuses. You can automate this process by using a requirements management tool.
3. Define Traceability Schema
Next, establish relationships between your artifacts using bidirectional links. These allow you to trace requirements both forward (to implementation and verification) and backward (to their origin or purpose).
Examples of Relationships include:
- Regulatory requirements ↔ Product requirements
- Product requirements ↔ Design artifacts
- Design artifacts ↔ Test cases
- Test cases ↔ Test results
- Requirements ↔ Defects/issues
- Code modules ↔ Requirements (via commit messages or tags)
Ensure every requirement is linked downstream (to design, code, and test cases) and that every issue can be traced upstream (to the failed test and impacted requirement).
4. Integrate Regulatory Metadata
If you operate in a regulated environment, now is the time to integrate the metadata around compliance requirements.
Each requirement should include:
- Compliance references (e.g., links to clauses or standards)
- Priority or criticality (e.g., Class A, safety-critical, risk data)
- Ownership (who is responsible)
- Compliance status (e.g., pending, verified, failed)
- Verification method (test, inspection, analysis, review)
Capturing this data early improves audit readiness and accountability.
5. Create Your Requirements Traceability Matrix
Once your artifacts and relationships are defined, you're ready to build your matrix. If you're using a manual approach (like Excel), structure your columns based on included artifacts:
- Column 1: Requirements
- Column 2: Tests
- Column 3: Test Results
- Column 4: Issues
Add your artifacts to the columns and map each requirement, its test cases, its issues, and its related artifacts.
Excel Requirement Traceability Matrix Example
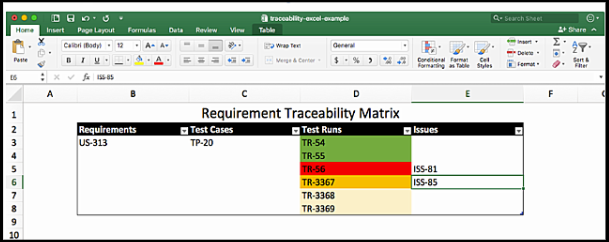
Want an easier way?
With Perforce ALM, you can create your traceability matrix with the click of a button.
Watch the on-demand demo to see how it works >>
6. Keep Your Matrix Up to Date
Traceability is only as reliable as your most recent matrix update. Enforce CI/CD and change management policies to ensure your matrix reflects the current state of the project and prevent defects or compliance failures.
Update your matrix every time:
- A requirement changes
- A test case is added, removed, or updated
- A defect is opened or resolved
Perform regular impact analyses to understand how changes impact compliance and artifact dependencies. Review your matrix often for accuracy and completeness.
7. Integrate Your Toolchain
Maintaining traceability manually in Excel doesn’t scale, and it leaves you vulnerable to human errors. Integrate with the right tools to maintain accuracy and save time, including:
- Test management tools
- Issue trackers
- Version control
With end-to-end integration and traceability automation, your matrix becomes a living system of record—providing a comprehensive record of your product’s entire development.
8. Perform Audits and Generate Reports
With a well-maintained matrix, you can quickly generate reports showing:
- Full traceability from regulation or requirement to implementation
- Unmet or failed requirements
- Test status summaries
- Issue statuses
Conduct regular peer reviews and traceability audits to confirm that links are accurate and complete. Maintain version history and change logs to support compliance and accountability.
Back to top3 Challenges of Not Using Traceability Matrix Tools
Creating your requirement traceability matrix in Excel or a platform other than a dedicated traceability tool is rarely worth the time or effort. Here are three reasons why:
1. Time
Creating a traceability matrix in Excel is not easy. It takes time to pull documentation together from disparate sources and even more time to tie it all together.
With busy workloads, responsibility for matrix creation often falls to the side. This can result in an incomplete traceability matrix that sows confusion around requirements and compromises your product quality.
2. Updates and Scalability
It's important that your traceability matrix always reflects the current statuses of your requirements, test cases, and issues. However, manually updating an excel document when tracking hundreds or even thousands of requirement changes, automated tests, and issues is near impossible. Even if your project starts small, you need a tool that can automatically handle these updates and scale if your project grows.
3. Compliance
If you aren’t certain that your traceability matrix is 100% accurate and up to date, you will have a difficult time demonstrating compliance or passing an audit. Meeting strict regulatory standards requires full traceability across requirements and their dependencies throughout your product’s development lifecycle.
Back to topWhy Requirement Traceability Tools are Better Than Spreadsheets
Using a dedicated traceability tool — such as Perforce ALM (formerly Helix ALM) — is faster, more efficient, and more reliable than using Excel to create a traceability matrix. Here’s why:
1. Automated Links and Dependencies
An automated tool adds the underlying links across the system that tie requirements to tests and issues. These relationships are automatically defined and updated as you go. That means your developers can focus on building the product instead of looking for and updating a spreadsheet.
2. A Single Source of Truth
You'll always have a current, comprehensive 'single pane of glass' view of your requirements and test coverage. You can easily publish reports and trace dependencies across the product lifecycle.
3.Risk and Compliance Management
With a traceability tool, all your artifacts are stored in a single location for easy review. Teams can explore the relationship between requirement and risk without sifting through layers of documents. Additionally, you can use your traceability matrix to validate that the product you’re shipping has been thoroughly tested and meets critical compliance goals, such as those required for a medical company.
Back to topVideo Tutorial: Sample Traceability Matrix in Perforce ALM
Watch how Perforce ALM streamlines the creation of a traceability matrix.
Back to topWant to see more? Our 20-minute on-demand demo shows you how Perforce ALM automates traceability and streamlines all aspects of your product workflow.
Access the demo now >>
9 Benefits of Creating Your Traceability Matrix with Perforce ALM
Perforce ALM is a configurable requirements management tool that offers comprehensive features to simply product development. Here are nine reasons users find Perforce the best solution for creating their traceability matrices:
- End-to-End Traceability Across the Stack
Perforce ALM excels at providing comprehensive, end-to-end traceability regardless of the tools you use in your development stack. Unlike some platforms that only offer traceability within their own suite, Perforce ALM captures and links requirements, tests, issues, and other artifacts across diverse tools and workflows. - Integrated Requirements, Test, and Issue Management
Perforce ALM is a modular suite that combines requirements management, test case management, and issue/defect tracking in a single platform. This eliminates the need for integration between separate tools. - Highly Configurable and Flexible Workflows
The platform offers high configurability, allowing you to adapt it to agile, waterfall, hybrid, or custom methodologies without forcing you to change your existing processes. You can customize workflows, fields, and relationships directly through an intuitive UI, making it easy to tailor the tool to your team's needs. - Automated Traceability and Impact Analysis
Perforce ALM automates the linking of requirements, tests, and issues, and provides built-in traceability matrix reports and impact analysis tools. Features like “Mark Suspects” proactively flag artifacts that may be affected by changes. - Collaboration and Visibility
Our platform enhances collaboration and visibility across teams and departments. Real-time updates, customizable dashboards, and advanced reporting features help keep all stakeholders informed and aligned throughout the entire development lifecycle. - Seamless Onboarding and Usability
Perforce ALM is designed for quick onboarding and ease of use, with ready-made templates and pre-configured reports. The Perforce support staff helps teams become productive faster and helps you troubleshoot when challenges arise. - Proven Compliance Support
Perforce ALM is widely used in highly regulated industries such as medical and automotive and supports compliance with standards like ISO 26262, ISO 14971, IEC 62304, and others. Its audit-ready traceability and reporting features simplify the process of proving compliance to regulatory bodies. - Integration with Popular DevOps Tools
The platform supports integration with leading development, automation, and CI/CD tools (e.g., Jira, Jenkins), ensuring that your ALM processes remain connected to the rest of your DevOps toolchain. - Cost-Effectiveness
Compared to legacy solutions, Perforce ALM is more affordable, and its modular approach allows you to license only the components you need. Plus, Perforce ALM is a scalable solution that grows with your company’s needs.
Create a Free Traceability Matrix with Perforce ALM
Using Perforce ALM, you can create an automated traceability matrix with the click of a button. You can then use your traceability matrix to prove compliance, improve product quality and safety, and understand the impact of change.
Try Perforce ALM today for free to see how easy it can be to create a traceability matrix and achieve end-to-end traceability. Watch our demo of Perforce ALM to see exactly how it:
- Automates traceability.
- Configures to any type of workflow.
- Automatically traces test cases back to requirements.
- Accommodates FMEA, ASIL, Hazard Analysis, and more with built-in risk tracking.
WATCH A DEMO TRY PERFORCE ALM FREE

